India's drugs regulator on Sunday approved Oxford COVID-19 vaccine Covishield, manufactured by the Serum Institute, and indigenously developed Covaxin of Bharat Biotech for restricted emergency use in the country, paving the way for a massive inoculation drive.
The approval by the Drugs Controller General of India (DCGI) was given on the basis of recommendations submitted by a COVID-19 subject expert committee (SEC) of the Central Drugs Standard Control Organisation (CDSCO).
"After adequate examination, CDSCO has decided to accept the recommendations of the Expert Committee and accordingly, vaccines of M/s Serum and M/s Bharat Biotech are being approved for restricted use in emergency situation," DCGI Dr VG Somani said.
This paves the way for the roll out of at least two vaccines in India in the coming days.
The Serum Institute of India, the world's largest vaccine manufacturer, has tied up with AstraZeneca to manufacture Covishield.
Covaxin has been indigenously developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR).
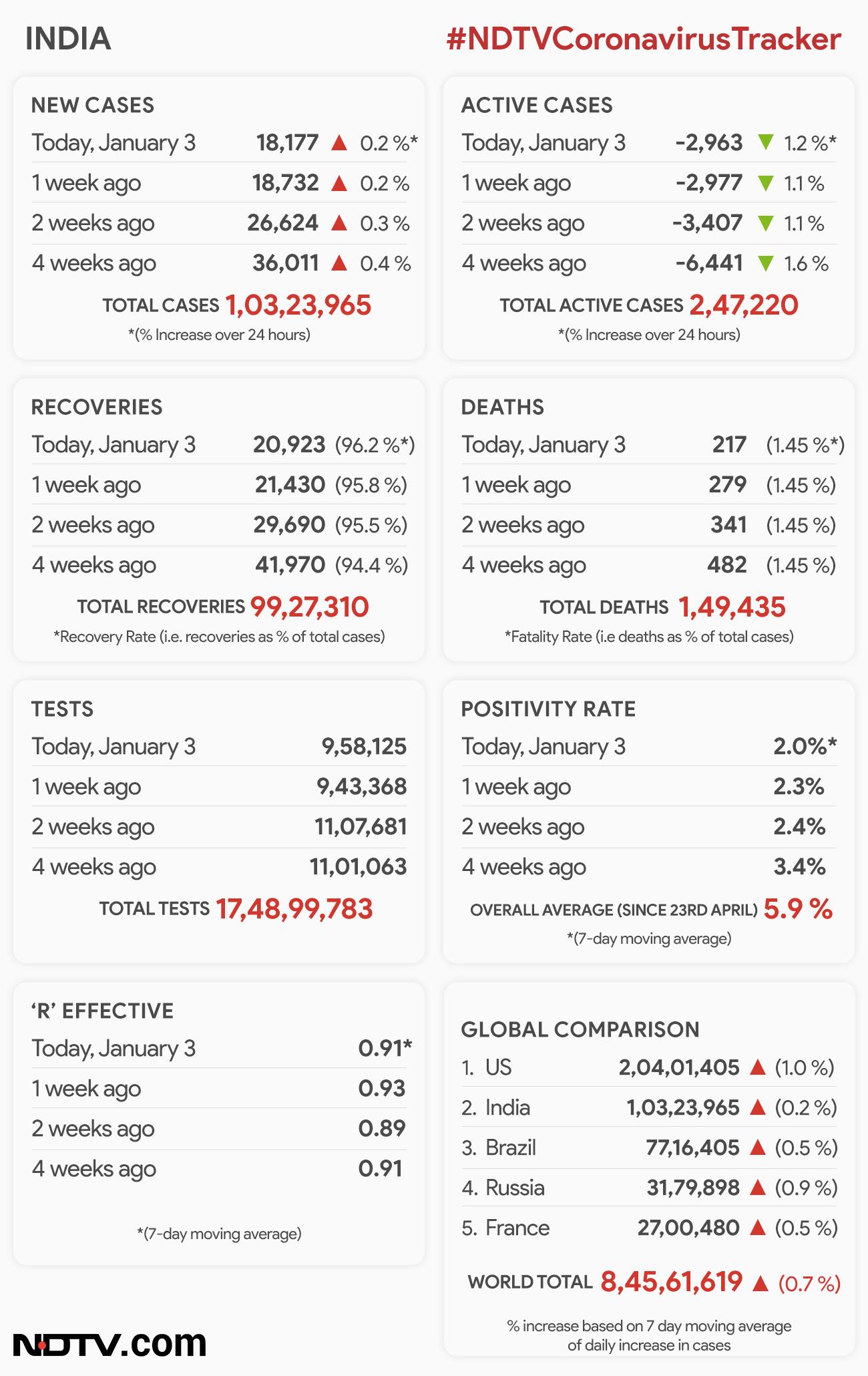
Meanwhile, India added 18,177 coronavirus infections in the past 24 hours, placing its overall number at 1.03 crore cases, the Health Ministry's data showed. The number of fresh infections is 4.7 per cent lower than on Saturday. In this period, India reported 217 deaths linked to the virus, taking the total number of fatalities to 1,49,435.
Here are the LIVE updates on Coronavirus Cases:
Bharat Biotech's coronavirus vaccine, which is still undergoing clinical trial, is likely to be the "back-up" vaccine in the coming days, Dr Randeep Guleria, the chief of Delhi's All-India Institute of Medical Sciences said today as two vaccines got emergency approval from the country's drug regulator.
Politics has started over the approval to Covaxin, with several opposition parties pointing out that the vaccine was approved without the full process.
Congress's Shashi Tharoor tweeted, "The Covaxin has not yet had Phase 3 trials. Approval was premature and could be dangerous. @drharshvardhan should please clarify. Its use should be avoided till full trials are over. India can start with the AstraZeneca vaccine in the meantime".
Congress leaders Shashi Tharoor and Jairam Ramesh, and Samajwadi Party chief Akhilesh Yadav had pointed out that Covaxin lacked efficacy data to show the vaccine's effectiveness before it was approved for emergency use.
"Disgraceful for anyone to politicise such a critical issue. Sh @ShashiTharoor, Sh @yadavakhilesh & Sh @Jairam_Ramesh don't try to discredit well laid out science-backed protocols followed for approving #COVID19 vaccines. Wake up & realise you are only discrediting yourselves," Harsh Vardhan tweeted.
Sixty-five people have been arrested from two illegal hookah bars in Delhi's Rohini after police raids. Coronavirus norms were also being violated at the bars, police said, saying social distancing was not followed at either establishment.
26 people, including four staff members, were arrested from Uptown Cafe in Rohini's Sector 9 on January 1. Ten hookahs have also been seized by the police in the raids.
35 people, including 10 women and three minors were arrested from Sector 8's Mud House restaurant in January 2. Six staff members were also arrested in the raid and 12 hookahs were confiscated as well. Three owners of the hookah bar have also been arrested in the raid.
Two top U.S. health officials on Sunday disputed a claim by President Donald Trump that federal data on COVID-19 cases and deaths in the United States is overblown, and both expressed optimism that the pace of vaccinations is picking up, Reuters reported.
"The deaths are real deaths," Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, said on ABC News' This Week, adding that jam-packed hospitals and stressed-out healthcare workers are "not fake. That's real."
With vaccine roll out on the cards after Drugs Controller General of India''s (DCGI) approval, the country needs to tackle public and administrative challenges to have a smooth vaccination roll-out, said Randeep Guleria, Director, All India Institute of Medical Sciences (AIIMS) on Sunday.
"The challenges are going to be both from the administration point of view and public point of view. From the administration, the challenge is to ensure that things run smoothly, and from point of view of public the challenge is to reassure them that this is a safe and effective vaccine," said Dr Guleria.
US officials on Sunday defended the stumbling campaign to vaccinate millions of Americans against the coronavirus, saying they expected much more to be done in coming weeks after delays.
"There have been a couple of glitches, that's understandable," top US scientist Anthony Fauci said on ABC. He said there would always be challenges in "trying to get a massive vaccine program started and getting off on the right foot."
Some 4.2 million Americans have received initial doses of the two-dose vaccines from Pfizer-BioNTech or Moderna, but that is far below official predictions of 20 million by the new year, AFP reported.
Egypt said Sunday it had opened an investigation into the deaths of four Covid-19 patients in an intensive care unit allegedly due to lack of oxygen, which had sparked a public outcry.
"The prosecutor's office in Al-Husseiniya (in the northern Sharqiya province) summoned the director of Al-Husseiniya hospital to question him over the deaths of four people due to lack of oxygen," a judicial source told AFP, without specifying the dates of the deaths.
Medical companies and shippers in Canada are racing to transport time-sensitive radiochemical materials used to treat cancer, as a pandemic-induced drop in passenger flights has narrowed transportation options and created cargo delays, Reuters reported.
Speaking after his weekly noon blessing, Francis said he had read newspaper reports of people catching flights to flee government curbs and seek fun elsewhere, Reuters reported.
The homes of the two highest-ranking members of the US Congress - House Speaker Nancy Pelosi and Senate Majority Leader Mitch McConnell - have been vandalised, police said, amid a political battle over a stimulus package to coronavirus-hit Americans. Fake blood and a severed pig''s head were reportedly left outside top Democrat Pelosi''s California house, which was also daubed with graffiti, PTI reported.
Delhi recorded 424 fresh COVID-19 cases, the lowest in over seven months, and 14 more fatalities due to the disease on Sunday, even as the positivity rate slipped to 0.62 per cent, authorities said. The COVID-19 case tally in the city stands at over 6.26 lakh and the death count due to the disease has climbed to 10,585, PTI reported.
Cases of COVID-19 in Britain are at record levels and increasing, fuelled by a new and more transmissible variant of the virus. That has already forced the government to cancel the planned reopening of schools in and around London, with calls from teaching unions for wider closures, Reuters reported.
Thailand, which had largely controlled the virus by mid-2020, saw a second wave of outbreaks beginning in December, Reuters reported.
"The Egyptian pharmaceutical authority approved on Saturday the Chinese Sinopharm vaccine," Hala Zayed said late Saturday, on the local MBC Masr channel.
The first batch of the vaccine was delivered in December, with further doses expected this month, AFP reported.
Israel said Sunday two million people will have received a two-dose Covid-19 vaccination by the end of January, a pace Prime Minister Benjamin Netanyahu boasts is the world's fastest.
Starting on December 19, when Netanyahu got his first jab, Israel launched an aggressive push to administer the vaccine made by US-German pharma alliance Pfizer-BioNTech.
Health Ministry Director General Hezi Levy said that because of the enthusiastic takeup, Israel would be easing the speed of vaccination to eke out stocks, AFP reported.
According to the news outlet, the new mutated strain was found in a family of four UK nationals, who are currently being quarantined at a private hospital, ANI reported.
"There is no doubt in my mind that schools are safe, and that education is a priority," he told the BBC.
Coronavirus vaccines will be given in the first phase to around nine lakh healthcare and frontline workers in the national capital, Delhi Health Minister Satyendra Jain on Sunday charted out the strategy of inoculation drive in the national capital, hours after two coronavirus vaccines got approval for emergency use in the country. Read more

Bharat Biotech's coronavirus vaccine, which is still undergoing clinical trial, is likely to be the "back-up" vaccine in the coming days, Dr Randeep Guleria, the chief of Delhi's All-India Institute of Medical Sciences said today as two vaccines got emergency approval from the country's drug regulator. Read more

India's Vaccination Process: All you need to know
- NDTV (@ndtv) January 3, 2021
Who will get the vaccine first? How can you register for the vaccine?
NDTV's Rishika Baruah breaks it down for you, answering all your Qs pic.twitter.com/5jqBzIH2P5


All the risks taken by Serum Institute have finally paid off, tweeted Adar Poonawalla, the chief executive of the Pune-based vaccine manufacturer, shortly after the country's drug regulator cleared its Covishield vaccine for emergency approval in India. Mr Poonawalla reiterated that the "Covishield" vaccine -- developed by Serum Institute of India in partnership with the Oxford University and pharma major AstraZeneca - is "safe and effective" against coronavirus and added that the vaccine "is ready to roll out in the coming weeks". Read more

WHO welcomes India's decision giving emergency use authorization to #COVID-19 #vaccines -
- WHO South-East Asia (@WHOSEARO) January 3, 2021
Dr Poonam Khetrapal Singh, Regional Director,
WHO South-East Asia Region pic.twitter.com/jyQGI6Gymp


Over 30 countries have reported cases of the highly-transmissible UK variant of the novel coronavirus, raising fears of increased global spread of the virus, even as countries begin to unroll vaccination programs in the new year. Read more