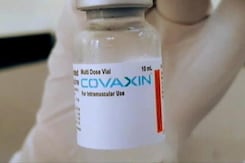
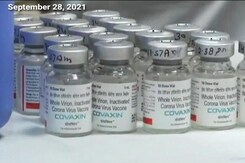
Covaxin Emergency Use Authorisation
- All
- News
- Videos
-

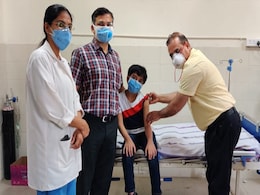
Covaxin Cleared For 6-12 Age Group, Corbevax For 5-12-Year-Olds
- Tuesday April 26, 2022
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The Drugs Controller General of India (DCGI) today gave emergency use authorisation to Bharat Biotech's Covaxin for children between the age group of 6-12 amid an uptick in the Covid cases in schools.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Cleared For Market Approval By Expert Panel
- Wednesday January 19, 2022
- India News | Press Trust of India
An expert panel of India's central drug authority today recommended granting regular market approval to Covishield and Covaxin, which are currently only authorised for emergency use in the country, subject to certain conditions, official sources said
-
 www.ndtv.com
www.ndtv.com
-

Recommendations On Covaxin Emergency Use For 2-18 Age Group Being Examined: Centre
- Tuesday November 30, 2021
- India News | Press Trust of India
The Covid Subject Expert Committee's recommendations on granting emergency use authorisation to Bharat Biotech's Covaxin for the 2-18 age group are being examined and additional information has been sought, Rajya Sabha was informed Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

WHO's Emergency Use Authorization For Covaxin "Expected Soon": Health Ministry
- Friday September 24, 2021
- India News | Asian News International
The Health Ministry on Friday hinted that the World Health Organization's (WHO) Emergency Use Authorization (EUA) for the COVID-19 vaccine, Covaxin, is expected soon.
-
 www.ndtv.com
www.ndtv.com
-

WHO Emergency Approval For Covaxin Delayed Till October 5
- Saturday September 18, 2021
- India News | Asian News International
World Health Organisation's (WHO) approval for the emergency use authorisation (EUA) to COVID-19 vaccine Covaxin, developed by the Hyderabad-based Bharat Biotech, is likely to be delayed till October 5.
-
 www.ndtv.com
www.ndtv.com
-

After Halting Emergency Use, Brazil Shelves Import Permit Of Covaxin
- Thursday July 29, 2021
- India News | Press Trust of India
After suspending the proposed clinical trials of Bharat Biotech's COVID-19 vaccine Covaxin and a request for Emergency Use Authorisation, Brazil now has suspended its decision to import four million doses of the jab into that country.
-
 www.ndtv.com
www.ndtv.com
-

Brazil Scraps Covaxin Emergency Use Application Amid Political Row: Report
- Tuesday July 27, 2021
- India News | Press Trust of India
After suspending the proposed clinical trials of Bharat Bioetch's Covaxin, Brazil has now scrapped the Emergency Use Authorisation request made by the Indian firm for the vaccine.
-
 www.ndtv.com
www.ndtv.com
-

We Respect US Decision On Covaxin's Emergency Use Authorisation: Centre
- Friday June 11, 2021
- India News | Asian News International
Centre said that the publication of Covaxin's phase 3 trial will be done sometime in 7-8 days.
-
 www.ndtv.com
www.ndtv.com
-

US Delays Use Of India-Made COVID-19 Vaccine Covaxin. What It Recommended
- Friday June 11, 2021
- World News | Press Trust of India
In a setback to Bharat Biotech's COVID-19 vaccine Covaxin, the US Food and Drug Administration has "recommended" Ocugen Inc, the US partner of the Indian vaccine maker, to go for Biologics Licence Application (BLA) route with additional data.
-
 www.ndtv.com
www.ndtv.com
-

Mexico Grants Authorisation For Emergency Use Of India's Covaxin Vaccine
- Wednesday April 7, 2021
- India News | ANI
The Mexico Federal Commission for the Protection Against Sanitary Risk (Cofepris) on Wednesday has granted authorization for the emergency use of India's first domestically-produced COVID-19 vaccine, Covaxin.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Safe, Side-Effects Negligible: NITI Aayog Member
- Tuesday January 12, 2021
- India News | ANI
Covishield and Covaxin, the two COVID-19 vaccines which have received Emergency Use Authorisation have been tested on thousands of people and side-effects are negligible, Dr VK Paul, Member (Health), NITI Aayog, said on Tuesday and noted that the two "are safest of the vaccines".
-
 www.ndtv.com
www.ndtv.com
-

4 New Units To Manufacture 700 Million Vaccine Doses A Year: Bharat Biotech
- Monday January 4, 2021
- India News | Press Trust of India
Bharat Biotech, which has been accorded Emergency Use Authorisation from the drug regulator for its COVID-19 vaccine Covaxin, today said they are setting up four vaccine manufacturing facilities with a combined capacity of 700 million doses per year.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Triggers Immune Response, No Adverse Effects In Phase 1 Trial
- Wednesday December 16, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Chandrashekar Srinivasan
Covaxin, which is one of three coronavirus vaccine candidates being considered for emergency use authorisation in India, induced an immune response and registered no serious adverse events, interim findings of Phase I trials have revealed
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Seeks Emergency Use Approval For Covaxin
- Tuesday December 8, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Stela Dey
Bharat Biotech, the Hyderabad-based pharmaceutical firm that has been developing coronavirus vaccine Covaxin, has applied to the central drug regulator seeking emergency use authorisation, sources said on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Cleared For 6-12 Age Group, Corbevax For 5-12-Year-Olds
- Tuesday April 26, 2022
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The Drugs Controller General of India (DCGI) today gave emergency use authorisation to Bharat Biotech's Covaxin for children between the age group of 6-12 amid an uptick in the Covid cases in schools.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Cleared For Market Approval By Expert Panel
- Wednesday January 19, 2022
- India News | Press Trust of India
An expert panel of India's central drug authority today recommended granting regular market approval to Covishield and Covaxin, which are currently only authorised for emergency use in the country, subject to certain conditions, official sources said
-
 www.ndtv.com
www.ndtv.com
-

Recommendations On Covaxin Emergency Use For 2-18 Age Group Being Examined: Centre
- Tuesday November 30, 2021
- India News | Press Trust of India
The Covid Subject Expert Committee's recommendations on granting emergency use authorisation to Bharat Biotech's Covaxin for the 2-18 age group are being examined and additional information has been sought, Rajya Sabha was informed Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

WHO's Emergency Use Authorization For Covaxin "Expected Soon": Health Ministry
- Friday September 24, 2021
- India News | Asian News International
The Health Ministry on Friday hinted that the World Health Organization's (WHO) Emergency Use Authorization (EUA) for the COVID-19 vaccine, Covaxin, is expected soon.
-
 www.ndtv.com
www.ndtv.com
-

WHO Emergency Approval For Covaxin Delayed Till October 5
- Saturday September 18, 2021
- India News | Asian News International
World Health Organisation's (WHO) approval for the emergency use authorisation (EUA) to COVID-19 vaccine Covaxin, developed by the Hyderabad-based Bharat Biotech, is likely to be delayed till October 5.
-
 www.ndtv.com
www.ndtv.com
-

After Halting Emergency Use, Brazil Shelves Import Permit Of Covaxin
- Thursday July 29, 2021
- India News | Press Trust of India
After suspending the proposed clinical trials of Bharat Biotech's COVID-19 vaccine Covaxin and a request for Emergency Use Authorisation, Brazil now has suspended its decision to import four million doses of the jab into that country.
-
 www.ndtv.com
www.ndtv.com
-

Brazil Scraps Covaxin Emergency Use Application Amid Political Row: Report
- Tuesday July 27, 2021
- India News | Press Trust of India
After suspending the proposed clinical trials of Bharat Bioetch's Covaxin, Brazil has now scrapped the Emergency Use Authorisation request made by the Indian firm for the vaccine.
-
 www.ndtv.com
www.ndtv.com
-

We Respect US Decision On Covaxin's Emergency Use Authorisation: Centre
- Friday June 11, 2021
- India News | Asian News International
Centre said that the publication of Covaxin's phase 3 trial will be done sometime in 7-8 days.
-
 www.ndtv.com
www.ndtv.com
-

US Delays Use Of India-Made COVID-19 Vaccine Covaxin. What It Recommended
- Friday June 11, 2021
- World News | Press Trust of India
In a setback to Bharat Biotech's COVID-19 vaccine Covaxin, the US Food and Drug Administration has "recommended" Ocugen Inc, the US partner of the Indian vaccine maker, to go for Biologics Licence Application (BLA) route with additional data.
-
 www.ndtv.com
www.ndtv.com
-

Mexico Grants Authorisation For Emergency Use Of India's Covaxin Vaccine
- Wednesday April 7, 2021
- India News | ANI
The Mexico Federal Commission for the Protection Against Sanitary Risk (Cofepris) on Wednesday has granted authorization for the emergency use of India's first domestically-produced COVID-19 vaccine, Covaxin.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Safe, Side-Effects Negligible: NITI Aayog Member
- Tuesday January 12, 2021
- India News | ANI
Covishield and Covaxin, the two COVID-19 vaccines which have received Emergency Use Authorisation have been tested on thousands of people and side-effects are negligible, Dr VK Paul, Member (Health), NITI Aayog, said on Tuesday and noted that the two "are safest of the vaccines".
-
 www.ndtv.com
www.ndtv.com
-

4 New Units To Manufacture 700 Million Vaccine Doses A Year: Bharat Biotech
- Monday January 4, 2021
- India News | Press Trust of India
Bharat Biotech, which has been accorded Emergency Use Authorisation from the drug regulator for its COVID-19 vaccine Covaxin, today said they are setting up four vaccine manufacturing facilities with a combined capacity of 700 million doses per year.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Triggers Immune Response, No Adverse Effects In Phase 1 Trial
- Wednesday December 16, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Chandrashekar Srinivasan
Covaxin, which is one of three coronavirus vaccine candidates being considered for emergency use authorisation in India, induced an immune response and registered no serious adverse events, interim findings of Phase I trials have revealed
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Seeks Emergency Use Approval For Covaxin
- Tuesday December 8, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Stela Dey
Bharat Biotech, the Hyderabad-based pharmaceutical firm that has been developing coronavirus vaccine Covaxin, has applied to the central drug regulator seeking emergency use authorisation, sources said on Monday.
-
 www.ndtv.com
www.ndtv.com