Covaxin Emergency Use Listing
- All
- News
- Videos
-

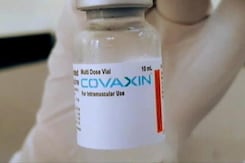
Covaxin Granted Emergency Use Listing In 13 Nations As On Jan 31 According To WHO: Government
- Friday February 4, 2022
- India News | Press Trust of India
Covaxin has been granted Emergency Use Listing (EUL) in 13 countries as on January 31 according to the WHO, Minister of State for Health Bharati Pravin Pawar informed the Lok Sabha on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Recognised By 96 Countries, Says Health Minister
- Wednesday November 10, 2021
- India News | Asian News International
Union Health Minister Mansukh Mandaviya said on Tuesday that 96 countries have recognised Covaxin and Covishield and both these COVID-19 vaccines have received emergency use listing (EUL) from the World Health Organisation (WHO).
-
 www.ndtv.com
www.ndtv.com
-

UK To Recognise Covaxin, No Quarantine For Fully Vaccinated Travellers
- Monday November 8, 2021
- India News | Reuters
Britain said it would recognise COVID-19 vaccines on the World Health Organization's Emergency Use Listing later this month, adding China's Sinovac, Sinopharm and India's Covaxin to the country's approved list of vaccines for inbound travellers.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Live Highlights: India Records 12,885 New Covid Cases In 24 Hours
- Friday November 5, 2021
- India News | NDTV News Desk
The World Health Organization has granted Bharat Biotech's Covaxin an Emergency Use Listing, said sources on Wednesday evening, which means the COVID-19 vaccine will now be recognised by other countries.
-
 www.ndtv.com
www.ndtv.com
-

"Covaxin Didn't Take Longest": WHO Clears Air On India vs China Claim
- Wednesday November 3, 2021
- India News | Reported by Vishnu Som
Covaxin, by no means, took the longest to get the World Health Organization's (WHO) approval for Emergency Use Listing, Chief Scientist Dr Soumya Swaminathan said today
-
 www.ndtv.com
www.ndtv.com
-

WHO In Talks With Covaxin Maker Bharat Biotech To Join Its Tech Access Pool: Official
- Wednesday November 3, 2021
- India News | Press Trust of India
The World Health Organisation is in negotiations with Bharat Biotech, whose COVID-19 vaccine Covaxin was given Emergency Use Listing, to join the global health agency's technology access pool to share technology and know how, a top WHO official said
-
 www.ndtv.com
www.ndtv.com
-

Approval To Covaxin Expands Availability Of Vaccines, Says WHO Official
- Wednesday November 3, 2021
- India News | Press Trust of India
The Emergency Use Listing nod to Bharat Biotech's Covaxin, which was found to have 78 per cent efficacy against COVID-19 of any severity, expands the availability of vaccines, the most effective medical tools to end the pandemic, a WHO official said
-
 www.ndtv.com
www.ndtv.com
-

Glad To See India's Covaxin Get Emergency Use Approval: WHO Chief
- Wednesday November 3, 2021
- World News | Edited by NDTV Newsdesk
World Health Organisation Director-General Tedros Adhanom Ghebreyesus on Wednesday said he is "glad" to see Covaxin get the Emergency Use Listing approval.
-
 www.ndtv.com
www.ndtv.com
-

What Covaxin-Maker Bharat Biotech Said After WHO Approved India-Made Jab
- Wednesday November 3, 2021
- India News | Edited by Sumana Nandy
The World Health organisation's approval of Covaxin will expedite global access and availability of the first India-made vaccine against coronavirus worldwide, its manufacturer Bharat Biotech said in a statement.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech's Covaxin Gets WHO Approval: The Journey So Far
- Wednesday November 3, 2021
- India News | Edited by NDTV Newsdesk
WHO on Wednesday "granted Covaxin an Emergency Use Listing (EUL)".
-
 www.ndtv.com
www.ndtv.com
-

WHO Clearance To Covaxin: What It Means
- Thursday November 4, 2021
- India News | Reported by Vishnu Som, Edited by Shatabdi Chowdhury
Covaxin, Bharat Biotech's COVID-19 vaccine has been granted Emergency Use Listing status by a World Health Organisation panel today
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Gets WHO Approval, Finally
- Wednesday November 3, 2021
- India News | Reported by Vishnu Som, Edited by Chandrashekar Srinivasan
The WHO has granted Covaxin an emergency use listing, sources said Wednesday, which means the 'made-in-India' vaccine will be recognised by other countries and Indians who received the shot need not self-quarantine or face restrictions abroad.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Highlights: WHO Approves Bharat Biotech's Covaxin For Emergency Use
- Thursday November 4, 2021
- India News | NDTV News Desk
The World Health Organization has granted Bharat Biotech's Covaxin an Emergency Use Listing, said sources on Wednesday evening, which means the COVID-19 vaccine will now be recognised by other countries.
-
 www.ndtv.com
www.ndtv.com
-

WHO Panel To Decide On Covaxin Emergency Use On Wednesday
- Tuesday November 2, 2021
- India News | Asian News International
The Technical Advisory Group of World Health Organisation has sought additional clarifications from Bharat Biotech, the manufacturer of Covaxin, in order to conduct a final Emergency Use Listing risk-benefit assessment for global use of the vaccine.
-
 www.ndtv.com
www.ndtv.com
-

No Nod For Covaxin Yet, WHO Seeks "Additional Clarifications"
- Wednesday October 27, 2021
- India News | Press Trust of India
The World Health Organisation's technical advisory group on Tuesday sought "additional clarifications" from Bharat Biotech for its COVID-19 vaccine Covaxin to conduct a final "risk-benefit assessment" for Emergency Use Listing of the vaccine.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Granted Emergency Use Listing In 13 Nations As On Jan 31 According To WHO: Government
- Friday February 4, 2022
- India News | Press Trust of India
Covaxin has been granted Emergency Use Listing (EUL) in 13 countries as on January 31 according to the WHO, Minister of State for Health Bharati Pravin Pawar informed the Lok Sabha on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Covishield, Covaxin Recognised By 96 Countries, Says Health Minister
- Wednesday November 10, 2021
- India News | Asian News International
Union Health Minister Mansukh Mandaviya said on Tuesday that 96 countries have recognised Covaxin and Covishield and both these COVID-19 vaccines have received emergency use listing (EUL) from the World Health Organisation (WHO).
-
 www.ndtv.com
www.ndtv.com
-

UK To Recognise Covaxin, No Quarantine For Fully Vaccinated Travellers
- Monday November 8, 2021
- India News | Reuters
Britain said it would recognise COVID-19 vaccines on the World Health Organization's Emergency Use Listing later this month, adding China's Sinovac, Sinopharm and India's Covaxin to the country's approved list of vaccines for inbound travellers.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Live Highlights: India Records 12,885 New Covid Cases In 24 Hours
- Friday November 5, 2021
- India News | NDTV News Desk
The World Health Organization has granted Bharat Biotech's Covaxin an Emergency Use Listing, said sources on Wednesday evening, which means the COVID-19 vaccine will now be recognised by other countries.
-
 www.ndtv.com
www.ndtv.com
-

"Covaxin Didn't Take Longest": WHO Clears Air On India vs China Claim
- Wednesday November 3, 2021
- India News | Reported by Vishnu Som
Covaxin, by no means, took the longest to get the World Health Organization's (WHO) approval for Emergency Use Listing, Chief Scientist Dr Soumya Swaminathan said today
-
 www.ndtv.com
www.ndtv.com
-

WHO In Talks With Covaxin Maker Bharat Biotech To Join Its Tech Access Pool: Official
- Wednesday November 3, 2021
- India News | Press Trust of India
The World Health Organisation is in negotiations with Bharat Biotech, whose COVID-19 vaccine Covaxin was given Emergency Use Listing, to join the global health agency's technology access pool to share technology and know how, a top WHO official said
-
 www.ndtv.com
www.ndtv.com
-

Approval To Covaxin Expands Availability Of Vaccines, Says WHO Official
- Wednesday November 3, 2021
- India News | Press Trust of India
The Emergency Use Listing nod to Bharat Biotech's Covaxin, which was found to have 78 per cent efficacy against COVID-19 of any severity, expands the availability of vaccines, the most effective medical tools to end the pandemic, a WHO official said
-
 www.ndtv.com
www.ndtv.com
-

Glad To See India's Covaxin Get Emergency Use Approval: WHO Chief
- Wednesday November 3, 2021
- World News | Edited by NDTV Newsdesk
World Health Organisation Director-General Tedros Adhanom Ghebreyesus on Wednesday said he is "glad" to see Covaxin get the Emergency Use Listing approval.
-
 www.ndtv.com
www.ndtv.com
-

What Covaxin-Maker Bharat Biotech Said After WHO Approved India-Made Jab
- Wednesday November 3, 2021
- India News | Edited by Sumana Nandy
The World Health organisation's approval of Covaxin will expedite global access and availability of the first India-made vaccine against coronavirus worldwide, its manufacturer Bharat Biotech said in a statement.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech's Covaxin Gets WHO Approval: The Journey So Far
- Wednesday November 3, 2021
- India News | Edited by NDTV Newsdesk
WHO on Wednesday "granted Covaxin an Emergency Use Listing (EUL)".
-
 www.ndtv.com
www.ndtv.com
-

WHO Clearance To Covaxin: What It Means
- Thursday November 4, 2021
- India News | Reported by Vishnu Som, Edited by Shatabdi Chowdhury
Covaxin, Bharat Biotech's COVID-19 vaccine has been granted Emergency Use Listing status by a World Health Organisation panel today
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Gets WHO Approval, Finally
- Wednesday November 3, 2021
- India News | Reported by Vishnu Som, Edited by Chandrashekar Srinivasan
The WHO has granted Covaxin an emergency use listing, sources said Wednesday, which means the 'made-in-India' vaccine will be recognised by other countries and Indians who received the shot need not self-quarantine or face restrictions abroad.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Highlights: WHO Approves Bharat Biotech's Covaxin For Emergency Use
- Thursday November 4, 2021
- India News | NDTV News Desk
The World Health Organization has granted Bharat Biotech's Covaxin an Emergency Use Listing, said sources on Wednesday evening, which means the COVID-19 vaccine will now be recognised by other countries.
-
 www.ndtv.com
www.ndtv.com
-

WHO Panel To Decide On Covaxin Emergency Use On Wednesday
- Tuesday November 2, 2021
- India News | Asian News International
The Technical Advisory Group of World Health Organisation has sought additional clarifications from Bharat Biotech, the manufacturer of Covaxin, in order to conduct a final Emergency Use Listing risk-benefit assessment for global use of the vaccine.
-
 www.ndtv.com
www.ndtv.com
-

No Nod For Covaxin Yet, WHO Seeks "Additional Clarifications"
- Wednesday October 27, 2021
- India News | Press Trust of India
The World Health Organisation's technical advisory group on Tuesday sought "additional clarifications" from Bharat Biotech for its COVID-19 vaccine Covaxin to conduct a final "risk-benefit assessment" for Emergency Use Listing of the vaccine.
-
 www.ndtv.com
www.ndtv.com