Covaxin Human Trial
- All
- News
- Videos
-

Covaxin Crosses Half-Way Mark In Phase-3 Trials, Says Bharat Biotech
- Tuesday December 22, 2020
- India News | Reported by Vishnu Som, Edited by Harish Pullanoor
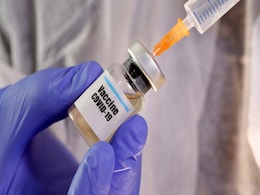
India's first and only phase III trial of a COVID-19 vaccine has crossed 13,000 subjects - the half-way mark - and proceeding successfully across multiple sites in India, Bharat Biotech, the maker of the inoculant Covaxin, announced today. The trials in the first and second phases had led to promising results.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech's COVID-19 Vaccine 'Covaxin' Commences Phase-3 Trial At AIIMS: Report
- Thursday November 26, 2020
- India News | Press Trust of India
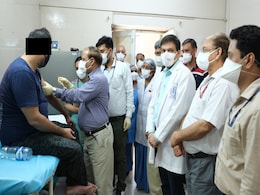
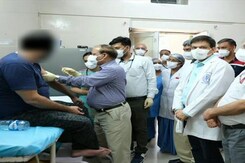
Phase-three of human clinical trial of the India-made COVID-19 vaccine candidate Covaxin began at AIIMS in Delhi on Thursday with Dr MV Padma Srivastava, the chief of Neurosciences Centre at the premier institute, and three other volunteers receiving the first dose.
-
 www.ndtv.com
www.ndtv.com
-

Phase-3 Trial Of Covaxin Begins In Odisha Institute
- Friday November 20, 2020
- India News | Press Trust of India
The third phase human trial of Covaxin, the much-awaited indigenously developed COVID-19 vaccine, has begun at an institute here, a senior official said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Third Phase Human Trial Of Covid-19 Vaccine To Begin In Bhubaneswar Soon
- Monday October 26, 2020
- India News | Press Trust of India
The third phase of the human trial of the indigenous vaccine against COVID-19, COVAXIN, will commence at a private hospital soon, an official said.
-
 www.ndtv.com
www.ndtv.com
-

In Odisha, Preparation On For 2nd Phase Of Human Clinical Trial Of Covid Vaccine
- Monday August 31, 2020
- India News | Press Trust India
Preparations are underway at a hospital in Bhubaneswar for the commencement of the second phase of human clinical trial of "Covaxin", India's indigenous COVID-19 vaccine, officials said.
-
 www.ndtv.com
www.ndtv.com
-

Human Clinical Trial Of COVID-19 Vaccine "Covaxin" Begins At UP Hospital
- Friday July 31, 2020
- India News | Press Trust of India
The human clinical trial of indigenously developed Covaxin, a possible vaccine against coronavirus, began at a hospital here, officials said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Human Trial Of "Covaxin" Begins At SUM Hospital In Bhubaneswar
- Tuesday July 28, 2020
- India News | ANI
The human trial of India's first indigenous vaccine candidate "Covaxin" began at the Institute of Medical Sciences and SUM Hospital in Bhubaneswar on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Human Clinical Trial Of Covid Vaccine "Covaxin" Begins At Odisha Hospital
- Monday July 27, 2020
- India News | Press Trust of India
The human clinical trial of indigenously developed Covaxin, a possible vaccine against the novel coronavirus, began at an institute here on Monday, a senior official said.
-
 www.ndtv.com
www.ndtv.com
-

Man, 30, Gets First Dose Of India's Covid Vaccine As Human Trial Begins
- Sunday July 26, 2020
- India News | Reported by Parimal Kumar, Edited by Aman Dwivedi
A 30-year-old man was on Friday given the first dose of undertrial coronavirus vaccine named as COVAXIN in Delhi's All India Institute Of Medical Sciences.
-
 www.ndtv.com
www.ndtv.com
-

Man Receives First Dose Of Covaxin In Phase-I Of Human Trial At Delhi AIIMS
- Saturday July 25, 2020
- India News | Press Trust of India
The phase-I human clinical trial of India's first indigenously-developed vaccine against novel coronavirus, Covaxin, began at Delhi AIIMS on Friday and the first dose of the injection was administered to a man who is in his 30s.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Human Trials: Delhi's Dr Dangs Lab To Partner With Bharat Biotech
- Thursday July 23, 2020
- India News | Press Trust of India
A Delhi-based private laboratory, Dr Dangs Lab, has claimed that it has been selected as the central lab for human clinical trials of Covaxin, India's indigenous COVID-19 vaccine candidate. The vaccine is being developed and manufactured by Bharat Biotech in collaboration with ICMR and NIV, Pune.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Human Trials: Delhi's Dr Dangs Lab To Partner With Bharat Biotech
- Thursday July 23, 2020
- India News | Press Trust of India
A Delhi-based private laboratory, Dr Dangs Lab, has claimed that it has been selected as the central lab for human clinical trials of Covaxin, India's indigenous COVID-19 vaccine candidate. The vaccine is being developed and manufactured by Bharat Biotech in collaboration with ICMR and NIV, Pune.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Vaccine Human Trial Begins, Result In 3 Months: AIIMS Head
- Monday July 20, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Nandini Gupta
The human trials of the indigenously developed coronavirus vaccine, COVAXIN, began today, AIIMS-Delhi Director Dr Randeep Guleria said, adding that it would take at least three months for researchers to arrive at the first set of data.
-
 www.ndtv.com
www.ndtv.com
-

AIIMS Panel Allows Human Trial Of India's First Coronavirus Vaccine
- Sunday July 19, 2020
- India News | Press Trust of India
The AIIMS Ethics Committee on Saturday gave its nod for a human clinical trial of the indigenously developed COVID-19 vaccine candidate Covaxin following which the premier hospital is likely to begin the exercise by enrolling healthy volunteers from Monday.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Starts Human Trials Of Anti-Covid Vaccine In Haryana
- Friday July 17, 2020
- India News | Edited by Edited by Nandini Gupta (with PTI inputs)
The human trial of India's first anti-COVID vaccine - Covaxin, which is being developed by Bharat Biotech - started at Rohtak's Post-Graduate Institute of Medical Sciences today, Haryana Health and Home Minister Anil Vij said.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Crosses Half-Way Mark In Phase-3 Trials, Says Bharat Biotech
- Tuesday December 22, 2020
- India News | Reported by Vishnu Som, Edited by Harish Pullanoor
India's first and only phase III trial of a COVID-19 vaccine has crossed 13,000 subjects - the half-way mark - and proceeding successfully across multiple sites in India, Bharat Biotech, the maker of the inoculant Covaxin, announced today. The trials in the first and second phases had led to promising results.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech's COVID-19 Vaccine 'Covaxin' Commences Phase-3 Trial At AIIMS: Report
- Thursday November 26, 2020
- India News | Press Trust of India
Phase-three of human clinical trial of the India-made COVID-19 vaccine candidate Covaxin began at AIIMS in Delhi on Thursday with Dr MV Padma Srivastava, the chief of Neurosciences Centre at the premier institute, and three other volunteers receiving the first dose.
-
 www.ndtv.com
www.ndtv.com
-

Phase-3 Trial Of Covaxin Begins In Odisha Institute
- Friday November 20, 2020
- India News | Press Trust of India
The third phase human trial of Covaxin, the much-awaited indigenously developed COVID-19 vaccine, has begun at an institute here, a senior official said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Third Phase Human Trial Of Covid-19 Vaccine To Begin In Bhubaneswar Soon
- Monday October 26, 2020
- India News | Press Trust of India
The third phase of the human trial of the indigenous vaccine against COVID-19, COVAXIN, will commence at a private hospital soon, an official said.
-
 www.ndtv.com
www.ndtv.com
-

In Odisha, Preparation On For 2nd Phase Of Human Clinical Trial Of Covid Vaccine
- Monday August 31, 2020
- India News | Press Trust India
Preparations are underway at a hospital in Bhubaneswar for the commencement of the second phase of human clinical trial of "Covaxin", India's indigenous COVID-19 vaccine, officials said.
-
 www.ndtv.com
www.ndtv.com
-

Human Clinical Trial Of COVID-19 Vaccine "Covaxin" Begins At UP Hospital
- Friday July 31, 2020
- India News | Press Trust of India
The human clinical trial of indigenously developed Covaxin, a possible vaccine against coronavirus, began at a hospital here, officials said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Human Trial Of "Covaxin" Begins At SUM Hospital In Bhubaneswar
- Tuesday July 28, 2020
- India News | ANI
The human trial of India's first indigenous vaccine candidate "Covaxin" began at the Institute of Medical Sciences and SUM Hospital in Bhubaneswar on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Human Clinical Trial Of Covid Vaccine "Covaxin" Begins At Odisha Hospital
- Monday July 27, 2020
- India News | Press Trust of India
The human clinical trial of indigenously developed Covaxin, a possible vaccine against the novel coronavirus, began at an institute here on Monday, a senior official said.
-
 www.ndtv.com
www.ndtv.com
-

Man, 30, Gets First Dose Of India's Covid Vaccine As Human Trial Begins
- Sunday July 26, 2020
- India News | Reported by Parimal Kumar, Edited by Aman Dwivedi
A 30-year-old man was on Friday given the first dose of undertrial coronavirus vaccine named as COVAXIN in Delhi's All India Institute Of Medical Sciences.
-
 www.ndtv.com
www.ndtv.com
-

Man Receives First Dose Of Covaxin In Phase-I Of Human Trial At Delhi AIIMS
- Saturday July 25, 2020
- India News | Press Trust of India
The phase-I human clinical trial of India's first indigenously-developed vaccine against novel coronavirus, Covaxin, began at Delhi AIIMS on Friday and the first dose of the injection was administered to a man who is in his 30s.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Human Trials: Delhi's Dr Dangs Lab To Partner With Bharat Biotech
- Thursday July 23, 2020
- India News | Press Trust of India
A Delhi-based private laboratory, Dr Dangs Lab, has claimed that it has been selected as the central lab for human clinical trials of Covaxin, India's indigenous COVID-19 vaccine candidate. The vaccine is being developed and manufactured by Bharat Biotech in collaboration with ICMR and NIV, Pune.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Human Trials: Delhi's Dr Dangs Lab To Partner With Bharat Biotech
- Thursday July 23, 2020
- India News | Press Trust of India
A Delhi-based private laboratory, Dr Dangs Lab, has claimed that it has been selected as the central lab for human clinical trials of Covaxin, India's indigenous COVID-19 vaccine candidate. The vaccine is being developed and manufactured by Bharat Biotech in collaboration with ICMR and NIV, Pune.
-
 www.ndtv.com
www.ndtv.com
-

Coronavirus Vaccine Human Trial Begins, Result In 3 Months: AIIMS Head
- Monday July 20, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Nandini Gupta
The human trials of the indigenously developed coronavirus vaccine, COVAXIN, began today, AIIMS-Delhi Director Dr Randeep Guleria said, adding that it would take at least three months for researchers to arrive at the first set of data.
-
 www.ndtv.com
www.ndtv.com
-

AIIMS Panel Allows Human Trial Of India's First Coronavirus Vaccine
- Sunday July 19, 2020
- India News | Press Trust of India
The AIIMS Ethics Committee on Saturday gave its nod for a human clinical trial of the indigenously developed COVID-19 vaccine candidate Covaxin following which the premier hospital is likely to begin the exercise by enrolling healthy volunteers from Monday.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Starts Human Trials Of Anti-Covid Vaccine In Haryana
- Friday July 17, 2020
- India News | Edited by Edited by Nandini Gupta (with PTI inputs)
The human trial of India's first anti-COVID vaccine - Covaxin, which is being developed by Bharat Biotech - started at Rohtak's Post-Graduate Institute of Medical Sciences today, Haryana Health and Home Minister Anil Vij said.
-
 www.ndtv.com
www.ndtv.com