Covid Vaccine Corbevax
- All
- News
- Videos
-

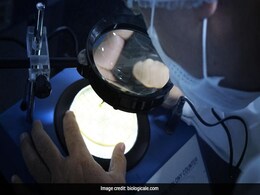
India's Corbevax Vaccine Gets WHO Emergency Use Listing Authorisation
- Tuesday January 16, 2024
- India News | Press Trust of India
India's COVID-19 vaccine, Corbevax, has been granted an Emergency Use Listing by the World Health Organisation, the company which manufactures it in India said Hyderabad today.
-
 www.ndtv.com
www.ndtv.com
-

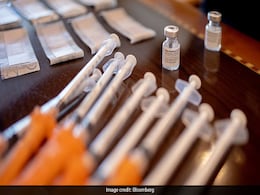
100 Million Corbevax Doses Supplied To Centre, Says Biological E
- Thursday August 11, 2022
- India News | Press Trust of India
Biological E. Ltd (BE) on Thursday said it has delivered 10 crore (100 million) doses of its COVID-19 vaccine, Corbevax, to the Centre so far.
-
 www.ndtv.com
www.ndtv.com
-

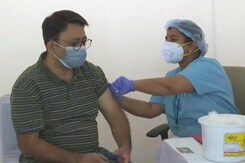
Corbevax Approved As Booster Dose For Adults Vaccinated With Covaxin, Covishield
- Wednesday August 10, 2022
- India News | Press Trust of India
The government on Wednesday approved Biological E's Corbevax as a precaution dose for those above 18 years fully vaccinated with either Covishield or Covaxin.
-
 www.ndtv.com
www.ndtv.com
-

Corbevax Cleared As Covid Booster Shot For Those 18 And Above
- Saturday June 4, 2022
- India News | Edited by Debanish Achom
Biological E's COVID-19 vaccine Corbevax has been cleared for booster shot for those 18 and above.
-
 www.ndtv.com
www.ndtv.com
-

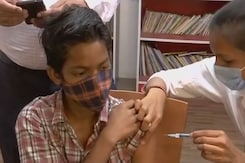
Vaccine For 12-14 Age Group: Delhi Parents Feel Relieved, Say Apt Move Ahead Of School Reopening
- Tuesday March 15, 2022
- Education | Reported by Press Trust of India
The COVID-19 vaccine to be administered to the 12-14 years age group would be Corbevax manufactured by Biological E. Limited, Hyderabad.
-
 www.ndtv.com/education
www.ndtv.com/education
-

Centre Releases Guidelines For Covid Vaccination Of 12-14 Age Group
- Tuesday March 15, 2022
- India News | Press Trust of India
The Centre today released guidelines for COVID-19 vaccination of children between 12-14 years that will begin from March 16 and said only Corbevax vaccine would be used for the beneficiaries of this age group.
-
 www.ndtv.com
www.ndtv.com
-

Influential US Congress Members Urge Vaccine Collaboration With India
- Saturday March 12, 2022
- India News | Press Trust of India
Members of America's influential Congressional Hispanic Caucus have urged US President Joe Biden to champion vaccine collaboration with India to bring an end to the COVID-19 pandemic.
-
 www.ndtv.com
www.ndtv.com
-

Biological E's Corbevax Is Second Covid Vaccine For Children In India
- Monday February 21, 2022
- India News | Edited by Debanish Achom
A second COVID-19 vaccine for children has received emergency use authorisation in India. Biological E Ltd's Corbevax has got EUA for children and teens between 12 and 18 years, the vaccine-maker said in a statement today.
-
 www.ndtv.com
www.ndtv.com
-

Pharma Stocks Gain After Centre Approves Use Of COVID Pill Molnupiravir
- Wednesday December 29, 2021
- Business | Edited by Nikita Prasad
Pharma Stocks Today: Pharmaceutical shares such as Cipla, Dr Reddy's Laboratories, Torrent Pharma, Sun Pharma Industries gained around two per cent each on Wednesday
-
 www.ndtv.com/business
www.ndtv.com/business
-

Biological E Aims To Produce 100 Million Doses Of Corbevax Every Month From Feb 2022
- Tuesday December 28, 2021
- India News | Press Trust of India
Biological E. Limited plans to complete production at a rate of 75 million doses of its COVID-19 vaccine Corbevax per month, anticipating over 100 million doses per month from February 2022.
-
 www.ndtv.com
www.ndtv.com
-

Will 2 New Vaccines Be Used As Boosters? Decision "Within Days"
- Tuesday December 28, 2021
- India News | Reported by Vishnu Som
The government will soon take a call whether the two new Covid vaccines approved today -- Corbevax and Covovax -- may be used for booster doses. Sources told NDTV that a decision is expected "within days".
-
 www.ndtv.com
www.ndtv.com
-

India's 2 New Covid Vaccines: What You Need To Know
- Tuesday December 28, 2021
- India News | Edited by NDTV Newsdesk
The two latest vaccines cleared by India are Corbevax and Covovax. The anti-viral drug Molnupiravir can be used during emergency.
-
 www.ndtv.com
www.ndtv.com
-

India Clears 2 New Vaccines And Merck's Covid Pill: 10 Points
- Tuesday December 28, 2021
- India News | Edited by Debanish Achom
The centre cleared two more vaccines today and one anti-viral drug to boost the fight against the COVID-19 pandemic. The two latest vaccines cleared by India are Corbevax and Covovax. The anti-viral drug Molnupiravir can be used during emergency.
-
 www.ndtv.com
www.ndtv.com
-

Hyderabad's Biological E Expects To Rollout Corbevax Covid Vaccine By November-End
- Monday October 25, 2021
- India News | Press Trust of India
Biological E. Limited (BE) is expecting its COVID-19 vaccine Corbevax to be rolled out by the end of November even as the city-based firm is getting ready with 100 million doses for the launch, Mahima Datla, Managing Director, BE said on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Biological E Seeks Regulator's Nod For Phase-3 Trial Of Booster Dose
- Tuesday October 12, 2021
- India News | Press Trust of India
Biological E has sought permission from India's drug regulator to conduct the phase-3 clinical trial for its COVID-19 vaccine Corbevax as a single booster dose for those who have been fully vaccinated with Covishield or Covaxin.
-
 www.ndtv.com
www.ndtv.com
-

India's Corbevax Vaccine Gets WHO Emergency Use Listing Authorisation
- Tuesday January 16, 2024
- India News | Press Trust of India
India's COVID-19 vaccine, Corbevax, has been granted an Emergency Use Listing by the World Health Organisation, the company which manufactures it in India said Hyderabad today.
-
 www.ndtv.com
www.ndtv.com
-

100 Million Corbevax Doses Supplied To Centre, Says Biological E
- Thursday August 11, 2022
- India News | Press Trust of India
Biological E. Ltd (BE) on Thursday said it has delivered 10 crore (100 million) doses of its COVID-19 vaccine, Corbevax, to the Centre so far.
-
 www.ndtv.com
www.ndtv.com
-

Corbevax Approved As Booster Dose For Adults Vaccinated With Covaxin, Covishield
- Wednesday August 10, 2022
- India News | Press Trust of India
The government on Wednesday approved Biological E's Corbevax as a precaution dose for those above 18 years fully vaccinated with either Covishield or Covaxin.
-
 www.ndtv.com
www.ndtv.com
-

Corbevax Cleared As Covid Booster Shot For Those 18 And Above
- Saturday June 4, 2022
- India News | Edited by Debanish Achom
Biological E's COVID-19 vaccine Corbevax has been cleared for booster shot for those 18 and above.
-
 www.ndtv.com
www.ndtv.com
-

Vaccine For 12-14 Age Group: Delhi Parents Feel Relieved, Say Apt Move Ahead Of School Reopening
- Tuesday March 15, 2022
- Education | Reported by Press Trust of India
The COVID-19 vaccine to be administered to the 12-14 years age group would be Corbevax manufactured by Biological E. Limited, Hyderabad.
-
 www.ndtv.com/education
www.ndtv.com/education
-

Centre Releases Guidelines For Covid Vaccination Of 12-14 Age Group
- Tuesday March 15, 2022
- India News | Press Trust of India
The Centre today released guidelines for COVID-19 vaccination of children between 12-14 years that will begin from March 16 and said only Corbevax vaccine would be used for the beneficiaries of this age group.
-
 www.ndtv.com
www.ndtv.com
-

Influential US Congress Members Urge Vaccine Collaboration With India
- Saturday March 12, 2022
- India News | Press Trust of India
Members of America's influential Congressional Hispanic Caucus have urged US President Joe Biden to champion vaccine collaboration with India to bring an end to the COVID-19 pandemic.
-
 www.ndtv.com
www.ndtv.com
-

Biological E's Corbevax Is Second Covid Vaccine For Children In India
- Monday February 21, 2022
- India News | Edited by Debanish Achom
A second COVID-19 vaccine for children has received emergency use authorisation in India. Biological E Ltd's Corbevax has got EUA for children and teens between 12 and 18 years, the vaccine-maker said in a statement today.
-
 www.ndtv.com
www.ndtv.com
-

Pharma Stocks Gain After Centre Approves Use Of COVID Pill Molnupiravir
- Wednesday December 29, 2021
- Business | Edited by Nikita Prasad
Pharma Stocks Today: Pharmaceutical shares such as Cipla, Dr Reddy's Laboratories, Torrent Pharma, Sun Pharma Industries gained around two per cent each on Wednesday
-
 www.ndtv.com/business
www.ndtv.com/business
-

Biological E Aims To Produce 100 Million Doses Of Corbevax Every Month From Feb 2022
- Tuesday December 28, 2021
- India News | Press Trust of India
Biological E. Limited plans to complete production at a rate of 75 million doses of its COVID-19 vaccine Corbevax per month, anticipating over 100 million doses per month from February 2022.
-
 www.ndtv.com
www.ndtv.com
-

Will 2 New Vaccines Be Used As Boosters? Decision "Within Days"
- Tuesday December 28, 2021
- India News | Reported by Vishnu Som
The government will soon take a call whether the two new Covid vaccines approved today -- Corbevax and Covovax -- may be used for booster doses. Sources told NDTV that a decision is expected "within days".
-
 www.ndtv.com
www.ndtv.com
-

India's 2 New Covid Vaccines: What You Need To Know
- Tuesday December 28, 2021
- India News | Edited by NDTV Newsdesk
The two latest vaccines cleared by India are Corbevax and Covovax. The anti-viral drug Molnupiravir can be used during emergency.
-
 www.ndtv.com
www.ndtv.com
-

India Clears 2 New Vaccines And Merck's Covid Pill: 10 Points
- Tuesday December 28, 2021
- India News | Edited by Debanish Achom
The centre cleared two more vaccines today and one anti-viral drug to boost the fight against the COVID-19 pandemic. The two latest vaccines cleared by India are Corbevax and Covovax. The anti-viral drug Molnupiravir can be used during emergency.
-
 www.ndtv.com
www.ndtv.com
-

Hyderabad's Biological E Expects To Rollout Corbevax Covid Vaccine By November-End
- Monday October 25, 2021
- India News | Press Trust of India
Biological E. Limited (BE) is expecting its COVID-19 vaccine Corbevax to be rolled out by the end of November even as the city-based firm is getting ready with 100 million doses for the launch, Mahima Datla, Managing Director, BE said on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Biological E Seeks Regulator's Nod For Phase-3 Trial Of Booster Dose
- Tuesday October 12, 2021
- India News | Press Trust of India
Biological E has sought permission from India's drug regulator to conduct the phase-3 clinical trial for its COVID-19 vaccine Corbevax as a single booster dose for those who have been fully vaccinated with Covishield or Covaxin.
-
 www.ndtv.com
www.ndtv.com