European Medicines Agency
- All
- News
- Videos
-

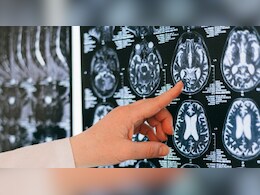
European Watchdog Partially Approves New Alzheimer's Drug
- Friday November 15, 2024
- World News | Agence France-Presse
Europe's medicines watchdog on Thursday partially approved a marketing request for a long-awaited new treatment for Alzheimer's disease, reversing an earlier decision not to give it the green light.
-
 www.ndtv.com
www.ndtv.com
-

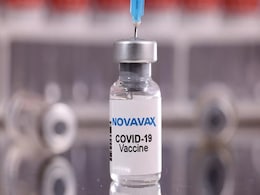
EU Regulator Backs Use Of Novavax Covid Shot As A Booster
- Thursday September 1, 2022
- World News | Reuters
The European Medicines Agency (EMA) on Thursday backed the use of Novavax's COVID-19 shot as a booster for adults, ahead of an anticipated rise in infections this winter.
-
 www.ndtv.com
www.ndtv.com
-

"No Time To Lose": EU's Nod To 2nd Covid Booster Jab For People Over 60
- Monday July 11, 2022
- World News | Agence France-Presse
The EU's health and medicine agencies said Monday they were recommending a second booster shot of a Covid vaccine for people over 60 years old, as infections rise again.
-
 www.ndtv.com
www.ndtv.com
-

EU Drug Regulator Reviews Use Of Smallpox Vaccine Against Monkeypox
- Tuesday June 28, 2022
- World News | Agence France-Presse
Monkeypox Virus: The European Union's drug regulator European Medicines Agency said it had started formally reviewing the use of a smallpox vaccine Imvanex to treat a growing number of cases of monkeypox.
-
 www.ndtv.com
www.ndtv.com
-

Pfizer Covid Pill Europe's First Approved Oral Treatment Against Covid
- Thursday January 27, 2022
- World News | Agence France-Presse
The EU's drug watchdog approved Pfizer's coronavirus pill on Thursday, making it the first oral antiviral treatment for the disease to be authorised in Europe.
-
 www.ndtv.com
www.ndtv.com
-

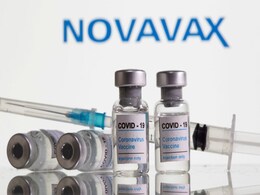
WHO Approves Novavax As 10th Authorised Covid Jab
- Tuesday December 21, 2021
- World News | Agence France-Presse
The World Health Organization on Tuesday approved a Covid vaccine made by US pharma giant Novavax for emergency use, after the European Union medicines regulator gave it the green light.
-
 www.ndtv.com
www.ndtv.com
-

Novavax Covid Vaccine Could Gain European, WHO Approval Next Week: Report
- Friday December 17, 2021
- World News | Reuters
Novavax's covid vaccine could receive approval from Europe's drug regulator next week and subsequently an emergency use listing from the WHO, the Financial Times reported on Thursday
-
 www.ndtv.com
www.ndtv.com
-

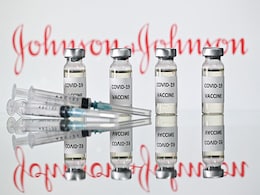
Johnson & Johnson Covid Vaccine Can Be Used As Booster: EU Medical Body
- Wednesday December 15, 2021
- World News | Agence France-Presse
The European Medicines Agency said Wednesday that Johnson & Johnson's Covid vaccine can be used as a booster shot two months after the first dose was administered, or after receiving other mRNA shots.
-
 www.ndtv.com
www.ndtv.com
-

Omicron Cases So Far Appear "Mostly Mild": European Medicines Agency
- Thursday December 9, 2021
- World News | Agence France-Presse
The European Medicines Agency (EMA) said Thursday that cases of Omicron so far appear to be "mostly mild", but cautioned it was still investigating whether the variant could cause severe disease.
-
 www.ndtv.com
www.ndtv.com
-

Too Early To Know If New Covid Variant Needs New Vaccine: EU Medical Body
- Friday November 26, 2021
- World News | Agence France-Presse
The EU's drug regulator said on Friday that it was closely monitoring the new B.1.1.529 Covid-19 variant, but it was too soon to tell if updated vaccines would be needed.
-
 www.ndtv.com
www.ndtv.com
-

Moderna Seeks EU Approval Of Covid Vaccine For 6 To 11 Year Olds
- Wednesday November 10, 2021
- World News | Agence France-Presse
Moderna has applied to the European Union's medicine regulator for approval of its Covid-19 vaccine for children aged six to 11, the US biotechnology company announced Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

European Union To Approve First Covid Antibody Drugs Amid Spike In Cases
- Tuesday November 9, 2021
- World News | Reuters
The European Union drugs regulator is set to authorise the use of two monoclonal antibodies to treat COVID-19 patients in coming days, two EU sources told Reuters, in its first approvals of such therapies.
-
 www.ndtv.com
www.ndtv.com
-

Pfizer Submits Data For Approval Of Covid Jab For Ages 5 To 11 In Europe
- Friday October 15, 2021
- World News | Agence France-Presse
Pfizer and BioNTech said Friday they had submitted data to the EU's medicines watchdog for the approval of their coronavirus vaccine for children aged 5 to 11, following a similar step in the United States.
-
 www.ndtv.com
www.ndtv.com
-

Nerve Disorder Listed As "Very Rare" Side Effect Of AstraZeneca Vaccine
- Thursday September 9, 2021
- World News | Agence France-Presse
The European Medicines Agency has listed the neurological disorder Guillain-Barre syndrome, which can cause temporary paralysis, as a "very rare" side effect of the AstraZeneca Covid-19 vaccine.
-
 www.ndtv.com
www.ndtv.com
-

No Application Received For Covishield Authorisation, Says EU Medical Body
- Saturday July 17, 2021
- India News | Reported by Vishnu Som, Edited by Ashutosh Tripathi
The European Medicines Agency (EMA) has not received any application from the Serum Institute of India for authorisation of Covishield, a COVID-19 vaccine.
-
 www.ndtv.com
www.ndtv.com
-

European Watchdog Partially Approves New Alzheimer's Drug
- Friday November 15, 2024
- World News | Agence France-Presse
Europe's medicines watchdog on Thursday partially approved a marketing request for a long-awaited new treatment for Alzheimer's disease, reversing an earlier decision not to give it the green light.
-
 www.ndtv.com
www.ndtv.com
-

EU Regulator Backs Use Of Novavax Covid Shot As A Booster
- Thursday September 1, 2022
- World News | Reuters
The European Medicines Agency (EMA) on Thursday backed the use of Novavax's COVID-19 shot as a booster for adults, ahead of an anticipated rise in infections this winter.
-
 www.ndtv.com
www.ndtv.com
-

"No Time To Lose": EU's Nod To 2nd Covid Booster Jab For People Over 60
- Monday July 11, 2022
- World News | Agence France-Presse
The EU's health and medicine agencies said Monday they were recommending a second booster shot of a Covid vaccine for people over 60 years old, as infections rise again.
-
 www.ndtv.com
www.ndtv.com
-

EU Drug Regulator Reviews Use Of Smallpox Vaccine Against Monkeypox
- Tuesday June 28, 2022
- World News | Agence France-Presse
Monkeypox Virus: The European Union's drug regulator European Medicines Agency said it had started formally reviewing the use of a smallpox vaccine Imvanex to treat a growing number of cases of monkeypox.
-
 www.ndtv.com
www.ndtv.com
-

Pfizer Covid Pill Europe's First Approved Oral Treatment Against Covid
- Thursday January 27, 2022
- World News | Agence France-Presse
The EU's drug watchdog approved Pfizer's coronavirus pill on Thursday, making it the first oral antiviral treatment for the disease to be authorised in Europe.
-
 www.ndtv.com
www.ndtv.com
-

WHO Approves Novavax As 10th Authorised Covid Jab
- Tuesday December 21, 2021
- World News | Agence France-Presse
The World Health Organization on Tuesday approved a Covid vaccine made by US pharma giant Novavax for emergency use, after the European Union medicines regulator gave it the green light.
-
 www.ndtv.com
www.ndtv.com
-

Novavax Covid Vaccine Could Gain European, WHO Approval Next Week: Report
- Friday December 17, 2021
- World News | Reuters
Novavax's covid vaccine could receive approval from Europe's drug regulator next week and subsequently an emergency use listing from the WHO, the Financial Times reported on Thursday
-
 www.ndtv.com
www.ndtv.com
-

Johnson & Johnson Covid Vaccine Can Be Used As Booster: EU Medical Body
- Wednesday December 15, 2021
- World News | Agence France-Presse
The European Medicines Agency said Wednesday that Johnson & Johnson's Covid vaccine can be used as a booster shot two months after the first dose was administered, or after receiving other mRNA shots.
-
 www.ndtv.com
www.ndtv.com
-

Omicron Cases So Far Appear "Mostly Mild": European Medicines Agency
- Thursday December 9, 2021
- World News | Agence France-Presse
The European Medicines Agency (EMA) said Thursday that cases of Omicron so far appear to be "mostly mild", but cautioned it was still investigating whether the variant could cause severe disease.
-
 www.ndtv.com
www.ndtv.com
-

Too Early To Know If New Covid Variant Needs New Vaccine: EU Medical Body
- Friday November 26, 2021
- World News | Agence France-Presse
The EU's drug regulator said on Friday that it was closely monitoring the new B.1.1.529 Covid-19 variant, but it was too soon to tell if updated vaccines would be needed.
-
 www.ndtv.com
www.ndtv.com
-

Moderna Seeks EU Approval Of Covid Vaccine For 6 To 11 Year Olds
- Wednesday November 10, 2021
- World News | Agence France-Presse
Moderna has applied to the European Union's medicine regulator for approval of its Covid-19 vaccine for children aged six to 11, the US biotechnology company announced Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

European Union To Approve First Covid Antibody Drugs Amid Spike In Cases
- Tuesday November 9, 2021
- World News | Reuters
The European Union drugs regulator is set to authorise the use of two monoclonal antibodies to treat COVID-19 patients in coming days, two EU sources told Reuters, in its first approvals of such therapies.
-
 www.ndtv.com
www.ndtv.com
-

Pfizer Submits Data For Approval Of Covid Jab For Ages 5 To 11 In Europe
- Friday October 15, 2021
- World News | Agence France-Presse
Pfizer and BioNTech said Friday they had submitted data to the EU's medicines watchdog for the approval of their coronavirus vaccine for children aged 5 to 11, following a similar step in the United States.
-
 www.ndtv.com
www.ndtv.com
-

Nerve Disorder Listed As "Very Rare" Side Effect Of AstraZeneca Vaccine
- Thursday September 9, 2021
- World News | Agence France-Presse
The European Medicines Agency has listed the neurological disorder Guillain-Barre syndrome, which can cause temporary paralysis, as a "very rare" side effect of the AstraZeneca Covid-19 vaccine.
-
 www.ndtv.com
www.ndtv.com
-

No Application Received For Covishield Authorisation, Says EU Medical Body
- Saturday July 17, 2021
- India News | Reported by Vishnu Som, Edited by Ashutosh Tripathi
The European Medicines Agency (EMA) has not received any application from the Serum Institute of India for authorisation of Covishield, a COVID-19 vaccine.
-
 www.ndtv.com
www.ndtv.com