Lamotrigine
- All
- News
- Videos
-

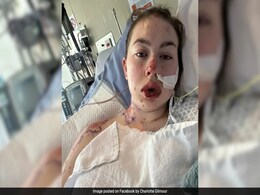
New Zealand Woman "Burned From The Inside" After Severe Reaction From Depression Medication
- Wednesday May 8, 2024
- Feature | Edited by Anjali Thakur
Ms Gilmour developed Steven-Johnson syndrome (SJS), a rare condition causing painful blisters on the skin, mouth, and oesophagus.
-
 www.ndtv.com
www.ndtv.com
-

Unichem Recalls 368 Bottles Of Anti-Epileptic Drug In US: Report
- Wednesday October 5, 2016
- Business | Press Trust of India
The tablets have been manufactured at the company's Goa facility. A single bottle contains 500 tablets of the medicine.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Dr Reddy's launches anti-epilepsy tablets in US market
- Wednesday June 26, 2013
- Business |
Drug firm Dr Reddy's Laboratories said on Wednesday that it has launched its generic Lamotrigine extended-release tablets in the American market following an approval by the US Food and Drug Administration.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Wockhardt gets approval to market anti-epilepsy tablets
- Tuesday January 8, 2013
- Business |
Drug firm Wockhardt today said it has received final approval from the US health regulator to market generic lamotrigine tablets - used for treatment of epilepsy - in America.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg. Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's La...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine
- Wednesday November 23, 2011
- Business | NDTV Correspondent
Lupin receives FDA approval for Lamotrigine tablets Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg. Lupi...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin Limited - Press Release
- Wednesday November 23, 2011
- Business | NDTV Correspondent
Lupin Limited has informed the Exchange regarding a Press Release dated June 4, 2010 titled "Lupin receives FDA approval for Lamotrigine tablets".
-
 www.ndtv.com/business
www.ndtv.com/business
-

New Zealand Woman "Burned From The Inside" After Severe Reaction From Depression Medication
- Wednesday May 8, 2024
- Feature | Edited by Anjali Thakur
Ms Gilmour developed Steven-Johnson syndrome (SJS), a rare condition causing painful blisters on the skin, mouth, and oesophagus.
-
 www.ndtv.com
www.ndtv.com
-

Unichem Recalls 368 Bottles Of Anti-Epileptic Drug In US: Report
- Wednesday October 5, 2016
- Business | Press Trust of India
The tablets have been manufactured at the company's Goa facility. A single bottle contains 500 tablets of the medicine.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Dr Reddy's launches anti-epilepsy tablets in US market
- Wednesday June 26, 2013
- Business |
Drug firm Dr Reddy's Laboratories said on Wednesday that it has launched its generic Lamotrigine extended-release tablets in the American market following an approval by the US Food and Drug Administration.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Wockhardt gets approval to market anti-epilepsy tablets
- Tuesday January 8, 2013
- Business |
Drug firm Wockhardt today said it has received final approval from the US health regulator to market generic lamotrigine tablets - used for treatment of epilepsy - in America.
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg. Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's La...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine tablets
- Friday November 25, 2011
- Business | NDTV Correspondent
Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg.Lupin's Lamotrigine is the AB-rated generic equivalent of GlaxoSmithKline's Lami...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin receives FDA approval for Lamotrigine
- Wednesday November 23, 2011
- Business | NDTV Correspondent
Lupin receives FDA approval for Lamotrigine tablets Lupin has announced that its U.S subsidiary, Lupin Pharmaceuticals Inc (LPI) has received final approval from U.S. Food and Drug Administration (USFDA) for its Abbreviated New Drug Application for its Lamotrigine tablets in strengths of 25 mg, 100 mg, 150 mg and 200 mg. Lupi...
-
 www.ndtv.com/business
www.ndtv.com/business
-
Lupin Limited - Press Release
- Wednesday November 23, 2011
- Business | NDTV Correspondent
Lupin Limited has informed the Exchange regarding a Press Release dated June 4, 2010 titled "Lupin receives FDA approval for Lamotrigine tablets".
-
 www.ndtv.com/business
www.ndtv.com/business