Us Pharma
- All
- News
- Videos
-

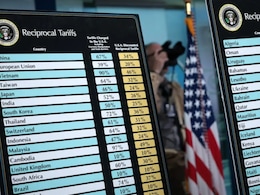
Trump Sets Stage For New Tariffs With Probe Into Pharma, Chip Imports
- Tuesday April 15, 2025
- World News | Edited by Sanstuti Nath
Amid US President Donald Trump's fresh tariff threats, the federal government in America has opened an investigation into the effects of importing certain pharmaceuticals and semiconductors on national security.
-
 www.ndtv.com
www.ndtv.com
-

US Opens Door To Tariffs On Pharma, Semiconductors
- Tuesday April 15, 2025
- World News | Agence France-Presse
The United States opened the door Monday to tariffs targeting high-end technology and pharmaceuticals, feeding the uncertainty gripping the global economy in a trade war that Chinese leader Xi Jinping warned can have "no winner."
-
 www.ndtv.com
www.ndtv.com
-

Pharma, Semi-conductors, Energy Products Exempted From Trump's Tariff
- Thursday April 3, 2025
- World News | Press Trust of India
Essential and strategic items such as pharmaceuticals, semiconductors, copper, and energy products like oil, gas, coal and LNG are exempted from the 26 per cent import duty announced by the US on Wednesday, according to think tank GTRI.
-
 www.ndtv.com
www.ndtv.com
-

With 2024 In Mind, Joe Biden's Big Move To Lower Drug Prices
- Wednesday August 30, 2023
- World News | Agence France-Presse
US President Joe Biden, who is campaigning for reelection with a heavy focus on easing voters' financial woes, on Tuesday launched a bid to lower the cost of certain prescription drugs -- a move Big Pharma pledged to continue battling in court.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Recalls 1,200 Bottles Of "Subpotent" Hypertension Drug In US
- Monday August 28, 2023
- India News | Press Trust of India
Glenmark Pharmaceuticals is recalling 1,200 bottles of a generic drug, used to treat high blood pressure, in the American market due to a manufacturing issue, according to the US Food and Drug Administration (USFDA).
-
 www.ndtv.com
www.ndtv.com
-

"Of Standard Quality": Government Sources On Eye Drop Flagged By US Body
- Tuesday April 4, 2023
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The samples of eye drops made by Chennai-based Global Pharma Healthcare - linked to three deaths and blindness in the US, are of "standard quality", sources in the Health Ministry told NDTV today.
-
 www.ndtv.com
www.ndtv.com
-

"No Contamination Found": Tamil Nadu Official As US Flags Indian Eye Drop
- Tuesday April 4, 2023
- India News | Reported by J Sam Daniel Stalin, Edited by Saikat Kumar Bose
Tamil Nadu's drug regulator has said it found "no contamination" in samples of eye drops manufactured by Chennai-based Global Pharma, which has hit headlines after a US watchdog linked cases of infections, blindness and even deaths to the eye drops.
-
 www.ndtv.com
www.ndtv.com
-

US Concerned Over Spread Of Drug-Resistant Germ Tied To Indian Eyedrops: Report
- Monday April 3, 2023
- World News | Edited by Debanish Achom
The top medical watchdog in the US has raised concerns over the likelihood of a highly drug-resistant bacteria, linked to eyedrops made by an Indian company, gaining a foothold in the US.
-
 www.ndtv.com
www.ndtv.com
-

Gujarat Pharma Firm Recalls Over 55,000 Medicine Bottles From US Market
- Sunday March 26, 2023
- India News | Press Trust of India
The affected lot is manufactured by Ahmedabad-based Zydus Lifesciences and marketed in the US by New Jersey-based Zydus Pharmaceuticals (USA) Inc.
-
 www.ndtv.com
www.ndtv.com
-

Alembic Pharma Gets Regulator's Approval To Market Antidepressant Drug In US
- Thursday March 9, 2023
- Business | Press Trust of India
Alembic Pharmaceuticals has received an approval from the US Food & Drug Administration to market Brexpiprazole tablets in strengths of 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg and 4 mg.
-
 www.ndtv.com/business
www.ndtv.com/business
-

Sun Pharma Recalls Over 34,000 Bottles Of Generic Drug In US After It Fails Test
- Saturday February 11, 2023
- India News | Press Trust of India
Drug major Sun Pharma is recalling over 34,000 bottles of a generic medication, used to treat high blood pressure, in the US market due to failed dissolution testing.
-
 www.ndtv.com
www.ndtv.com
-

Drug Regulatory Body Takes Samples Of Eye Drops Linked To Death In US
- Saturday February 4, 2023
- India News | Press Trust of India
Members of Tamil Nadu's Drug Controller and Central Drug Control Authority conducted a late-night inspection of manufacturing premises of Global Pharma Healthcare Private Limited.
-
 www.ndtv.com
www.ndtv.com
-

Late-Night Inspection At Chennai Firm Linked To Vision Loss, Death In US
- Saturday February 4, 2023
- India News | Written by J Sam Daniel Stalin, Edited by Akhil Kumar
Global Pharma Healthcare, the drug manufacturer at the centre of the controversy, has said it's working closely with the US Food and Drug Administration (FDA).
-
 www.ndtv.com
www.ndtv.com
-

Indian Firm Suspends Production Of Eye Drops Linked To Death In US
- Friday February 3, 2023
- India News | Reported by J Sam Daniel Stalin, Edited by Divyanshu Dutta Roy
The US Centers for Disease Control and Prevention (CDC) said it is testing unopened bottles of EzriCare Artificial Tears eye drops, manufactured by Chennai-based Global Pharma Healthcare.
-
 www.ndtv.com
www.ndtv.com
-

Trump Sets Stage For New Tariffs With Probe Into Pharma, Chip Imports
- Tuesday April 15, 2025
- World News | Edited by Sanstuti Nath
Amid US President Donald Trump's fresh tariff threats, the federal government in America has opened an investigation into the effects of importing certain pharmaceuticals and semiconductors on national security.
-
 www.ndtv.com
www.ndtv.com
-

US Opens Door To Tariffs On Pharma, Semiconductors
- Tuesday April 15, 2025
- World News | Agence France-Presse
The United States opened the door Monday to tariffs targeting high-end technology and pharmaceuticals, feeding the uncertainty gripping the global economy in a trade war that Chinese leader Xi Jinping warned can have "no winner."
-
 www.ndtv.com
www.ndtv.com
-

Pharma, Semi-conductors, Energy Products Exempted From Trump's Tariff
- Thursday April 3, 2025
- World News | Press Trust of India
Essential and strategic items such as pharmaceuticals, semiconductors, copper, and energy products like oil, gas, coal and LNG are exempted from the 26 per cent import duty announced by the US on Wednesday, according to think tank GTRI.
-
 www.ndtv.com
www.ndtv.com
-

With 2024 In Mind, Joe Biden's Big Move To Lower Drug Prices
- Wednesday August 30, 2023
- World News | Agence France-Presse
US President Joe Biden, who is campaigning for reelection with a heavy focus on easing voters' financial woes, on Tuesday launched a bid to lower the cost of certain prescription drugs -- a move Big Pharma pledged to continue battling in court.
-
 www.ndtv.com
www.ndtv.com
-

Glenmark Pharmaceuticals Recalls 1,200 Bottles Of "Subpotent" Hypertension Drug In US
- Monday August 28, 2023
- India News | Press Trust of India
Glenmark Pharmaceuticals is recalling 1,200 bottles of a generic drug, used to treat high blood pressure, in the American market due to a manufacturing issue, according to the US Food and Drug Administration (USFDA).
-
 www.ndtv.com
www.ndtv.com
-

"Of Standard Quality": Government Sources On Eye Drop Flagged By US Body
- Tuesday April 4, 2023
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The samples of eye drops made by Chennai-based Global Pharma Healthcare - linked to three deaths and blindness in the US, are of "standard quality", sources in the Health Ministry told NDTV today.
-
 www.ndtv.com
www.ndtv.com
-

"No Contamination Found": Tamil Nadu Official As US Flags Indian Eye Drop
- Tuesday April 4, 2023
- India News | Reported by J Sam Daniel Stalin, Edited by Saikat Kumar Bose
Tamil Nadu's drug regulator has said it found "no contamination" in samples of eye drops manufactured by Chennai-based Global Pharma, which has hit headlines after a US watchdog linked cases of infections, blindness and even deaths to the eye drops.
-
 www.ndtv.com
www.ndtv.com
-

US Concerned Over Spread Of Drug-Resistant Germ Tied To Indian Eyedrops: Report
- Monday April 3, 2023
- World News | Edited by Debanish Achom
The top medical watchdog in the US has raised concerns over the likelihood of a highly drug-resistant bacteria, linked to eyedrops made by an Indian company, gaining a foothold in the US.
-
 www.ndtv.com
www.ndtv.com
-

Gujarat Pharma Firm Recalls Over 55,000 Medicine Bottles From US Market
- Sunday March 26, 2023
- India News | Press Trust of India
The affected lot is manufactured by Ahmedabad-based Zydus Lifesciences and marketed in the US by New Jersey-based Zydus Pharmaceuticals (USA) Inc.
-
 www.ndtv.com
www.ndtv.com
-

Alembic Pharma Gets Regulator's Approval To Market Antidepressant Drug In US
- Thursday March 9, 2023
- Business | Press Trust of India
Alembic Pharmaceuticals has received an approval from the US Food & Drug Administration to market Brexpiprazole tablets in strengths of 0.25 mg, 0.5 mg, 1 mg, 2 mg, 3 mg and 4 mg.
-
 www.ndtv.com/business
www.ndtv.com/business
-

Sun Pharma Recalls Over 34,000 Bottles Of Generic Drug In US After It Fails Test
- Saturday February 11, 2023
- India News | Press Trust of India
Drug major Sun Pharma is recalling over 34,000 bottles of a generic medication, used to treat high blood pressure, in the US market due to failed dissolution testing.
-
 www.ndtv.com
www.ndtv.com
-

Drug Regulatory Body Takes Samples Of Eye Drops Linked To Death In US
- Saturday February 4, 2023
- India News | Press Trust of India
Members of Tamil Nadu's Drug Controller and Central Drug Control Authority conducted a late-night inspection of manufacturing premises of Global Pharma Healthcare Private Limited.
-
 www.ndtv.com
www.ndtv.com
-

Late-Night Inspection At Chennai Firm Linked To Vision Loss, Death In US
- Saturday February 4, 2023
- India News | Written by J Sam Daniel Stalin, Edited by Akhil Kumar
Global Pharma Healthcare, the drug manufacturer at the centre of the controversy, has said it's working closely with the US Food and Drug Administration (FDA).
-
 www.ndtv.com
www.ndtv.com
-

Indian Firm Suspends Production Of Eye Drops Linked To Death In US
- Friday February 3, 2023
- India News | Reported by J Sam Daniel Stalin, Edited by Divyanshu Dutta Roy
The US Centers for Disease Control and Prevention (CDC) said it is testing unopened bottles of EzriCare Artificial Tears eye drops, manufactured by Chennai-based Global Pharma Healthcare.
-
 www.ndtv.com
www.ndtv.com